| CLICK HERE FOR INDEX PAGE | |
THE HYDROGEN FUEL CELL, HYDROGEN POWERED CARS AND SECURITY LIGHTS - A SIMPLE EXPLANATION |
|
| PDF FILE - CLICK HERE FOR PRINTABLE WORKSHEET | |
|
A hydrogen car is one that uses the gas hydrogen as a
fuel source. There are basically two ways in which hydrogen is used: |
|
| THE EXHAUST OF A HDROGEN POWERED CAR - WATER IS THE ONLY BY-PRODUCT | |
 |
|
|
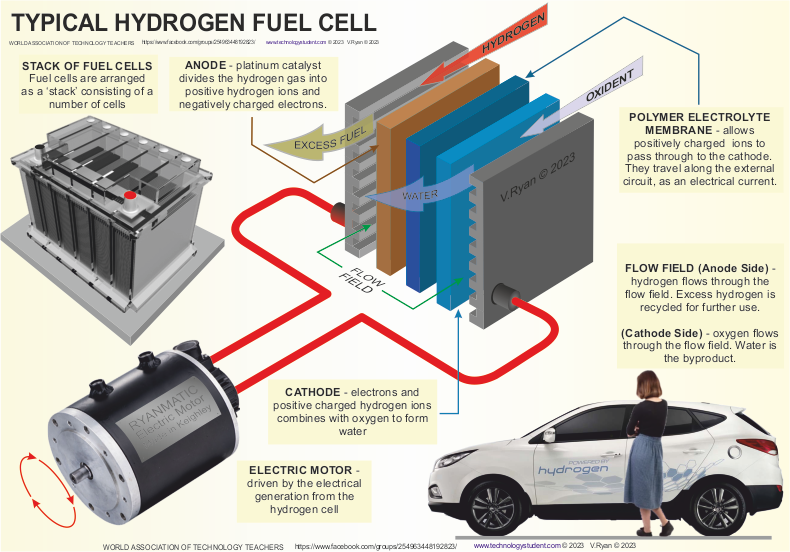
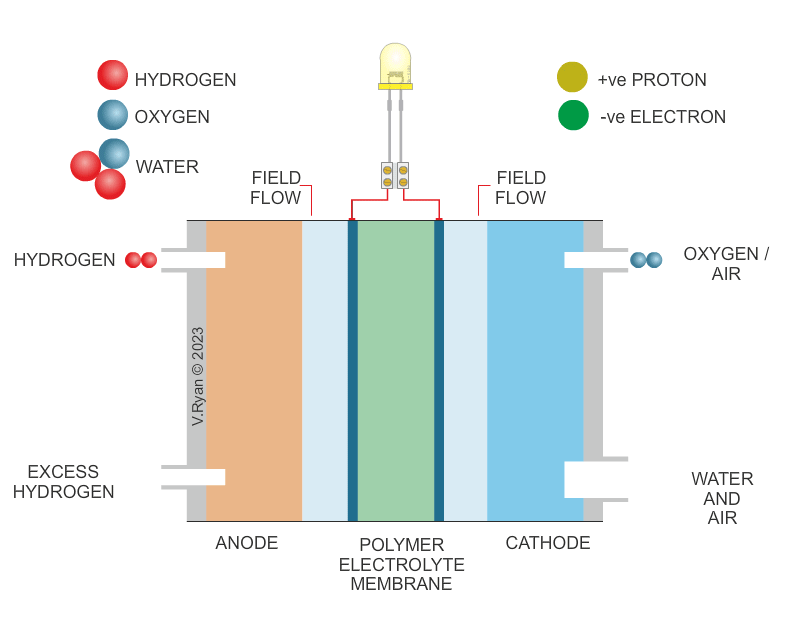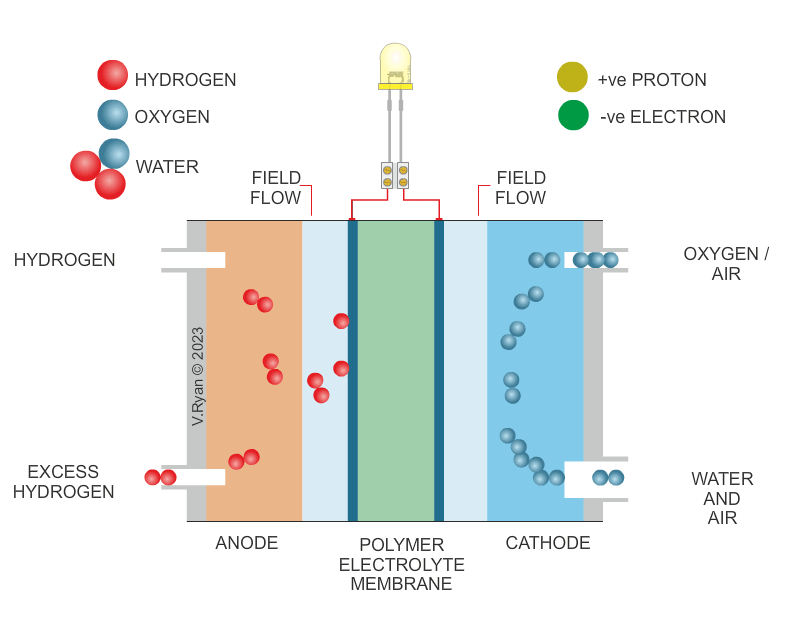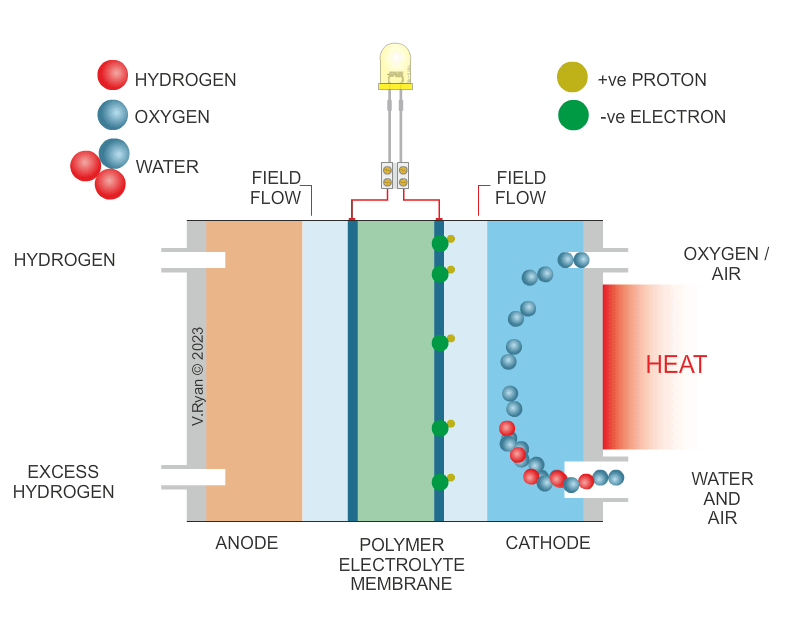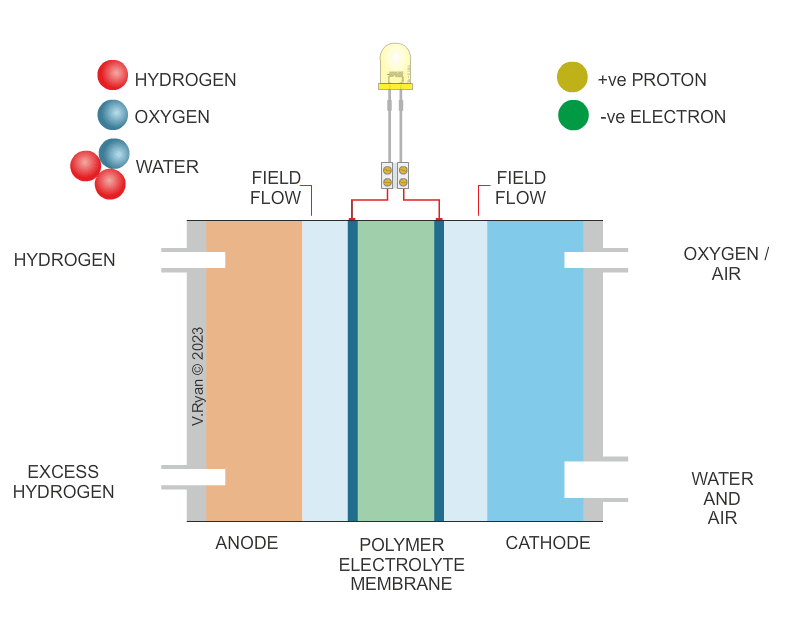
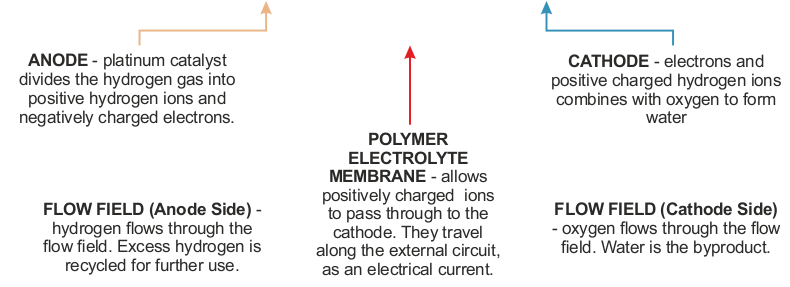
THE HYDROGEN FUEL CELL |
|
|
A fuel cell is an essential part of a hydrogen car
producing electricity to drive motors. It consists of three parts - the
ANODE, CATHODE
and CATALYST. |
|
 |
|
| HOW THE HYDROGEN FUEL CELL WORKS | |
 |
|
 |
|
 |
|
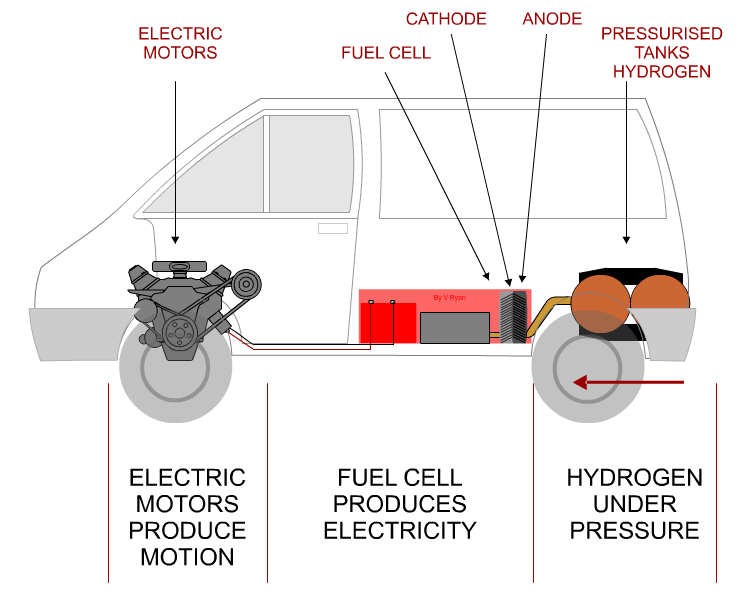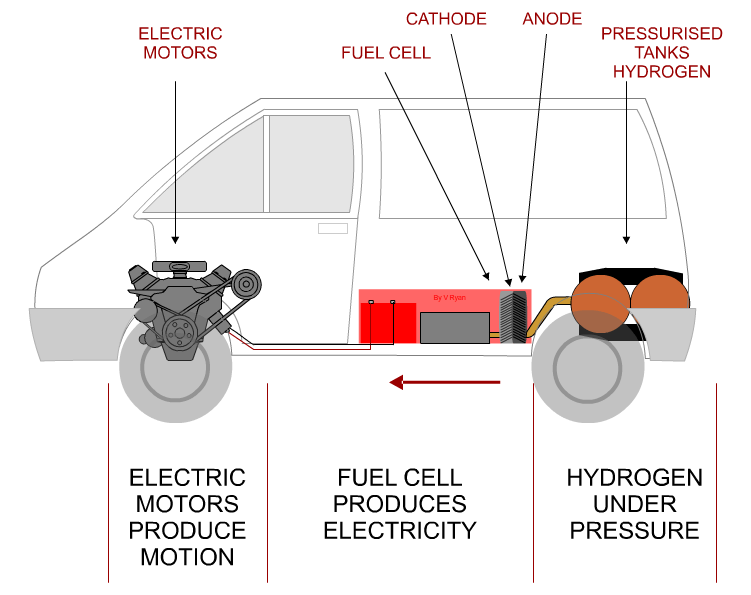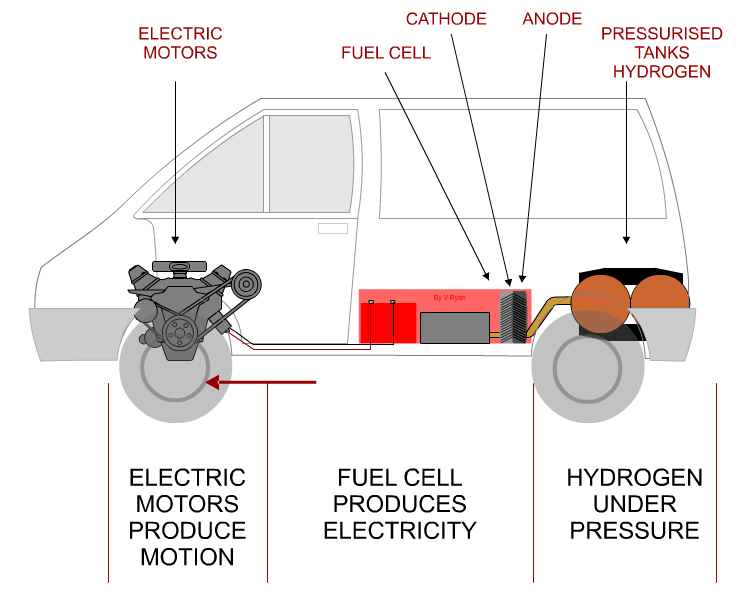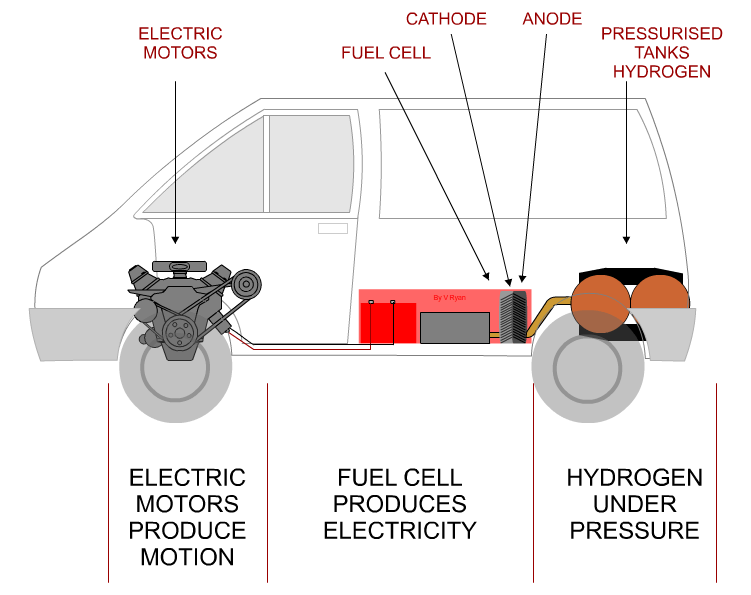
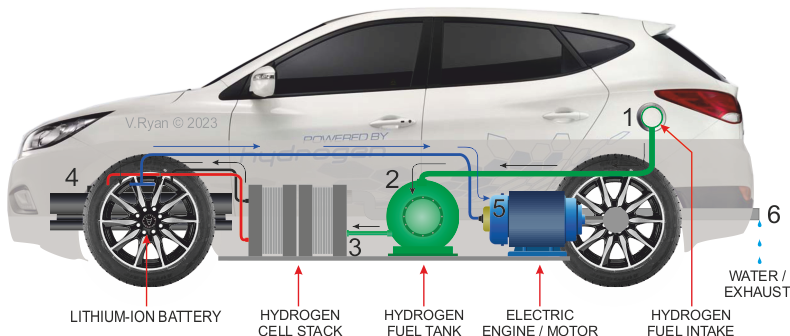
| MORE DETAILED DIAGRAM OF A HYDROGEN CAR | |
 |
|
| 1. HYDROGEN FUEL ADDED TO THE TANK. 2. HYDROGEN FLOWS FROM THE TANK, THROUGH FUEL CELL (3) 4. ELECTRICITY PRODUCED BY FUEL CELL STORED IN BATTERY AND ALSO FLOWS DIRECTLY TO THE ELECTRIC MOTOR (5), DRIVING THE CAR FORWARD. 6. WATER IS A BYPRODUCT - EXITS FROM THE EXHAUST |
|
| Hydrogen fuel cells are now used in low power systems, such as security lighting. They provide a cost effective option, especially in areas where there is no electricity source. They are also silent, unlike those powered by a generator. | |
 |
|
| CLICK HERE FOR TECHNOLOGY AND ENVIRONMENT INDEX PAGE | |