In industry, electroplating is the process whereby a cheap base metal, is coated with a much more expensive metal, in order to make it visually attractive and aesthetically pleasing (gold and silver plating are examples). Electroplating, is usually a decorative process and the aim is to increase the visual appeal of cheaper jewellery. It also serves to provide the surface, with a level of protection against corrosion. Some everyday products including bathroom taps, have been electroplated with chrome for decoration as well as corrosion resistance. Electroplating is also used to apply a conductive surface to metals, that are of low conductivity. Depending on the material to be applied as a coating, the opposite can also the achieved, increasing conductivity.
An example of copper plating is seen below. The process for electroplating with other metals is similar.
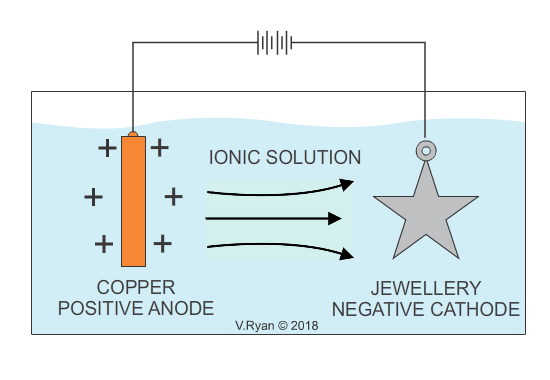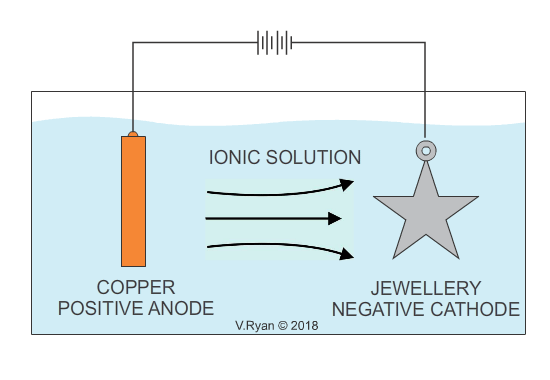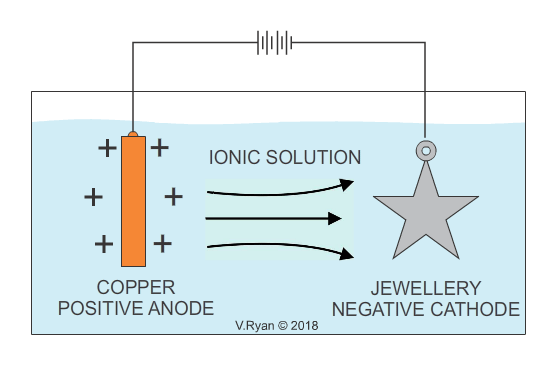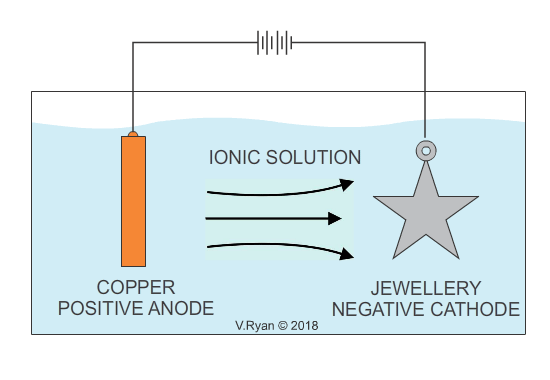
Also see - ELECTROPLATING - ELECTRO-GALVANISED
6. Galvanising Steel -
1
7. Galvanising Steel -
2
