In industry, electroplating is the process whereby a cheap base metal, is coated with a much more expensive metal, in order to make it visually attractive and aesthetically pleasing (gold and silver plating are examples). Electroplating is usually a decorative process and is often used to increase the visual appeal of cheaper jewellery. It also serves to provide the surface with a level of protection against corrosion. Some everyday products such as bathroom taps have been electroplated with chrome for decoration, as well as corrosion resistance. Electroplating is also used to apply a conductive surface to metals, that are of low conductivity or non-conductive.
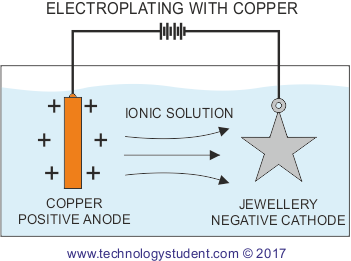
The copper anode is ionised once the electrical current is allowed to flow. The ionic solution allows the positively charged copper atoms to flow to the negatively charged base metal, where they are deposited on the surface. This produces an effective coating of copper.

ELECTROPLATING - 4

