GALVANISED?
Steel is a very strong and versatile metal used to manufacture a large range of products. Steel surrounds us in our every day lives, as it is used to construct buildings and structures. It is a versatile material, although it has one weakness, corrosion.
If an exposed steel surface comes in contact with water or moisture, rust can take hold. Rust can damage the surface of the steel as seen on corroded car bodywork. It is possible that on a large structure, such as a bridge, rust can cause structural failure leading to collapse.
Galvanised steel is steel that has been coated with zinc in order to prevent rusting / corrosion.
The hot dipping process applies quite a think layer of zinc to the steel, by passing the steel through a molten bath of zinc. The temperature of the zinc is usually in the region of 460 degrees centigrade. The zinc forms a bond with the steel by forming an iron-zinc alloy. The zinc also forms a zinc oxide when it comes in contact with the air which also helps prevent corrosion.

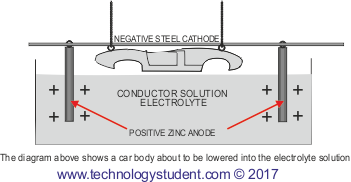
The steel car body is electrically charged as negative and is suspended in a conducting solution, known as the electrolyte. Rods of pure zinc are positively charged and it is the zinc from these rods that is eventually deposited on the surface of the steel. The rods are suspended in the electrolyte.
‘Top of the range’ cars often have galvanised steel bodies.